
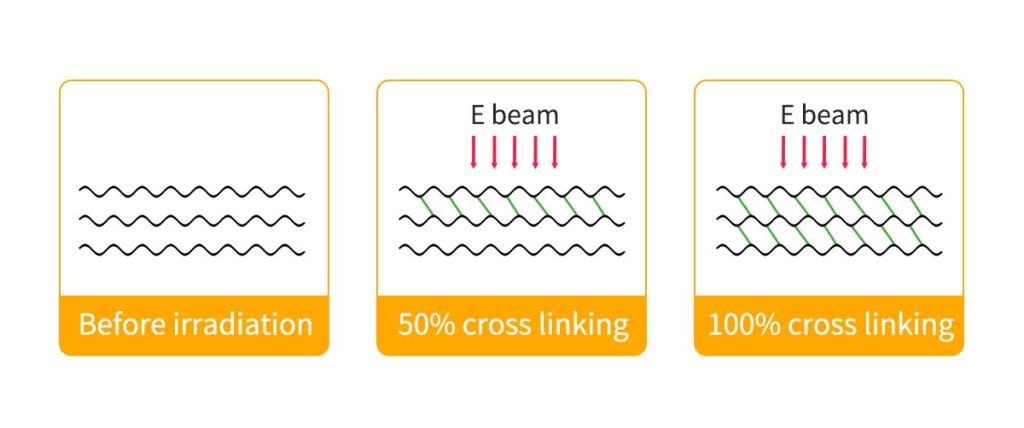
Crosslinking in electron beam processing breaks existing bonds and creates stronger links between polymer molecules, boosting resistance to heat and abrasion.
Chain scission breaks molecular bonds without reforming them, leading to controlled degradation and specific particle sizes.
The balance between these processes determines material properties. Processing conditions influence which effect dominates.
Key Takeaways
- Crosslinking strengthens polymers by creating durable bonds, enhancing heat and abrasion resistance.
- Chain scission breaks polymer chains into smaller segments, affecting properties like ductility and brittleness.
- Balancing crosslinking and chain scission during processing optimizes material performance for various industrial applications.
Electron Beam Processing
Process Overview
Electron beam processing uses high-energy electrons to modify the structure and properties of polymers. The process begins when technicians place the material, such as a composite preform, onto a mold. They seal the mold and infuse it with electron beam curing under vacuum. Uniform pressure consolidates the material. The next step involves irradiating the material with a high-intensity electron beam, which initiates curing and triggers molecular changes.
The main equipment parameters include energy, current, and line speed. Energy, measured in MeV, determines how deeply the beam penetrates the material. Current, measured in mA, affects the intensity of the electron beam. Line speed controls how quickly the material passes through the beam, impacting the irradiation dose.
| Parameter | Description |
|---|---|
| Energy | Measured in MeV, determines penetration ability of the beam. |
| Current | Measured in mA, affects intensity of the electron beam. |
| Line Speed | Speed at which the material passes through the electron beam, impacting irradiation dose. |
Electron beam technology also finds use in sterilization. The concentrated stream of electrons alters chemical bonds, damages DNA, and destroys microorganisms.
Material Modification
Electron beam processing changes polymers at the molecular level. Fast-moving electrons ionize long chain molecules, forming a crosslinked polymer matrix. This process improves heat resistance and creep resistance. When polymers like PEEK undergo irradiation, their crystallinity decreases, and new functional groups appear due to oxidation. Mechanical properties shift as chain scission and crystalline phase growth occur.
The absorbed dose, or ionizing energy delivered per unit mass, plays a crucial role in effective polymer modification. Different polymers respond in unique ways. For example, high-density polyethylene shows a 57% increase in hardness after exposure to 198 kGy, while low-density polyethylene increases by 24%. Polycarbonate’s decomposition temperature rises with dose, indicating improved stability.
Electron beam processing enhances elasticity, strength, and thermal stability in many materials. The interplay between crosslinking and chain scission determines the final properties.
Crosslinking Mechanism
How Does Crosslinking Occur?
Crosslinking begins when high-energy electrons from the electron beam interact with the polymer. The molecules absorb these electrons, which leads to the formation of free radicals. These free radicals appear on adjacent chains and cause hydrogen atoms to be removed. As a result, hydrogen gas is released during the crosslinking process. The polymer radicals then combine, creating a three-dimensional network that links the chains together.
The following table outlines the main steps in the crosslinking process:
| Step | Description |
|---|---|
| 1 | Polyethylene molecules absorb electrons, leading to the formation of free radicals. |
| 2 | Free radicals are formed on adjacent chains, resulting in hydrogen abstraction. |
| 3 | Hydrogen gas is liberated during the crosslinking process. |
| 4 | Polymer radicals combine to form a three-dimensional network. |
Different types of radicals play a role in e-beam crosslinking. Alkyl, allyl, and polyenyl radicals form through dehydrogenation and scission of polymer chains. These radicals recombine to create strong carbon-carbon and carbon-oxygen-carbon bonds. Carboxylic acid comonomers can also form crosslinks through C-O-C bonds, while peroxy radicals contribute to intermolecular crosslinking.
| Type of Radical | Formation Process | Crosslinking Mechanism |
|---|---|---|
| Alkyl, Allyl, Polyenyl | Produced by dehydrogenation and scission of irradiated polymer chains | Recombine to form C-C and C-O-C bonds |
| Carboxylic Acid Comonomers | Dehydrogenation of radicals | Forms crosslinks through C-O-C bonds |
| Peroxy Radicals | Produced during irradiation | Contributes to intermolecular crosslinking |
Some polymers show a higher tendency for crosslinking under electron beam exposure. Polyamides and polyethylene are especially susceptible. The chemical structure, crystallinity, and presence of cross-linking agents influence the degree of crosslinking. Polyamides can be cross-linked by both electron beam and gamma radiation. The presence of methyl groups in polyamides enhances mechanical properties, while higher water absorption improves the crosslinking process.
Crosslinking Effects
Crosslinking changes the physical and chemical properties of a material. The process creates a denser network within the polymer, which leads to lower water content and greater strength. The swelling ratio of protein polymers decreases, improving machinability and shape retention. The type and density of crosslinking affect mechanical properties. For example, glutaraldehyde crosslinking results in significant mechanical strength, while genipin produces less cytotoxic but weaker constructs.
The degree of crosslinking also impacts drug diffusion and release. Increased crosslinking density reduces the space between macromolecular chains, which slows drug release. Lower water content and swelling ratio further decrease the rate of drug diffusion. Crosslinking under confined conditions leads to slower drug release compared to crosslinking in solution.
The following table shows how crosslinker concentration affects polymer performance:
| Crosslinker Concentration (%) | Crosslinking Degree (%) | Impact Strength (kJ/m²) | Heat Distortion Temperature (°C) |
|---|---|---|---|
| 0.5 | 5.1 | N/A | N/A |
| 1.5 | 68.2 | N/A | N/A |
| 2.0 | 74.7 | N/A | N/A |
| 2.5 | 69 | 104.73 | 80.1 |
As the crosslinking degree increases, the material shows improved impact strength and higher heat distortion temperature. This means the polymer can withstand greater mechanical stress and higher temperatures without deforming. Crosslinking also increases molecular weight, which further enhances durability and stability.
Note: Crosslinking can affect cytocompatibility. Some crosslinkers, such as glutaraldehyde, may cause cytotoxicity and calcification. Choosing the right crosslinker is important for applications that require biocompatibility.
Chain Scission Mechanism
How Does Chain Scission Occur?
Chain scissioning describes a process where the molecular chains in a material break into smaller segments. During electron beam processing, high-energy electrons strike the polymer, causing bonds within the long molecular chains to break. This chain scissioning process does not require harsh chemicals, making it a cleaner and more controlled method for modifying materials.
- Chain scissioning in polymers results in the production of smaller molecular sub-units.
- The process leads to a reduction in molecular weight, which changes the physical properties of the material, such as ductility and brittleness.
- Electron beam processing can break down cellulose fibers from wood, making them suitable for biodegradable detergents.
- The technique also processes polytetrafluoroethylene (PTFE) to create fine powders used in inks and automotive coatings.
Some polymer structures are more prone to chain scissioning than others. Polypropylene (PP) often undergoes chain scissioning when exposed to electron beam irradiation. This process significantly affects the mechanical properties of PP. Poly(tetramethylene oxide) also shows a high tendency for chain scissioning, especially under electron beam or gamma irradiation. The microstructures of PTMG-PU are more susceptible to these effects compared to other polymers.
Tip: Chain scissioning can be used to tailor the size and solubility of polymer particles for specific industrial applications.
Chain Scission Effects
Chain scissioning changes the mechanical and chemical properties of a material in several important ways. When a chain breaks, it creates two dangling ends, which can alter the way the material behaves, even at low levels of chemical aging.
- Chain scissioning leads to a decrease in fracture toughness and ultimate elongation.
- The glass transition temperature (Tg) can increase as the number of chain scissions rises, especially when cross-link density is high.
- For polymers with a strong β transition, chain scissioning can increase the modulus plateau, a phenomenon called internal antiplasticization.
- Degraded networks formed by chain scissioning differ from ideal networks, often showing reduced fracture properties as crosslinking density increases.
The extent of chain scissioning influences both degradation and processability. The following table summarizes key effects:
| Evidence Description | Key Points |
|---|---|
| Chain scission reduces average molecular weight | Affects mechanical properties and processability |
| Shorter chains formed are more soluble | Leads to mass loss in the environment |
| Accumulation of acidic products | Accelerates degradation through autocatalysis |
In materials like low-density polyethylene (LDPE), chain fission, branching, crosslinking, and gel formation can occur at the same time. Chain fission dominates during the initial cycles of processing, while microgel formation becomes more important later.
Chain scissioning provides a valuable tool for engineers and scientists. By controlling the degree of chain scissioning, they can adjust the solubility, particle size, and degradation rate of a material. This control allows for the design of polymers with specific properties for targeted applications, such as biodegradable plastics or specialty powders.
Crosslinking vs. Chain Scission
Simultaneous Reactions
During electron beam processing, both crosslinking and chain scission can take place at the same time. Scientists observe these reactions in most polymers when exposed to high-energy electrons. Several indicators support this phenomenon:
- Both reactions occur together, with the balance depending on the polymer blend.
- Free radicals, shown by hydrogen radiation yield, signal active crosslinking.
- Shifts in weight-average and number-average molar mass confirm that both processes happen simultaneously.
- Environmental conditions and the structure of the polymer influence which reaction dominates.
- Oxidative degradation can increase chain scission, but crosslinking often remains the main process within the polymer itself.
Influencing Factors
The dominance of crosslinking or chain scission depends on several factors. The chemical structure of the polymer plays a key role. Long hydrocarbon chains and saturated carbon rings favor crosslinking, while aromatic rings can reduce both reactions. The presence of carbon-carbon double bonds also increases the chance of crosslinking. Processing conditions further affect the outcome:
| Processing Condition | Effect on Crosslinking and Chain Scission |
|---|---|
| Electron Beam Dose | Higher doses increase chain scission, which can make the material brittle. |
| Atmosphere | Nitrogen or vacuum reduces oxygen, limiting oxidative degradation. |
| Temperature | Careful control helps maintain the desired properties. |
Balancing these reactions is essential for achieving the right material properties. Crosslinking increases hardness, glass transition temperature, and durability. Chain scission, on the other hand, can lower hardness and lead to material degradation. Engineers must adjust processing conditions to create materials with the best performance for their intended use.
Applications
Industrial Uses of Crosslinking
Industries rely on crosslinking to improve the durability and performance of polymer products in everyday applications. Automotive manufacturers use crosslinked polymers for tires, gaskets, and hoses, which must withstand heat and mechanical stress. Electronics companies select crosslinked insulating materials for wires and cables, ensuring thermal stability and reliable electrical properties. Medical device makers choose crosslinked tubing and prosthetics for biocompatibility and resilience. Construction firms install pipes and insulation made from crosslinked polyethylene, benefiting from chemical resistance and long-lasting durability. Packaging producers create films and foams with enhanced barrier properties, making them ideal for food and protective wraps.
- Automotive: Tires, gaskets, hoses
- Electronics: Wire and cable insulation
- Medical: Tubing, catheters, prosthetics
- Construction: PEX pipes, insulation
- Packaging: Films, foams
Crosslinking increases tensile strength, abrasion resistance, and form stability. These improvements help products maintain integrity in everyday applications, even under extreme conditions.
Industrial Uses of Chain Scission
Chain scission plays a key role in recycling and specialty manufacturing. Food packaging industries use electron beam processing to sterilize products and produce aseptic packaging. Recycling facilities degrade PTFE to create lubricants, inks, and coatings. The table below shows how different industries apply chain scission:
| Industry | Purpose |
|---|---|
| Food Packaging | Sterilizing products, aseptic packaging |
| Recycling | Degrading PTFE for lubricants, inks, coatings |
Chain scission reduces molecular weight, increasing fluidity and processability. In everyday applications, this allows manufacturers to tailor polymer properties for specific needs.
Process Optimization
Engineers optimize electron beam parameters to balance crosslinking and chain scission for the best material performance. Adjusting beam current and voltage controls penetration depth and intensity. Low beam current minimizes charging and improves resolution, while optimal voltage ensures sufficient intensity without damaging surface details.
| Parameter | Effect |
|---|---|
| Low Beam Current | Minimizes charging, higher resolution |
| High Beam Voltage | Deep penetration, can blur surface details |
| Optimal Settings | Sufficient intensity, minimal damage |
Process optimization leads to improved hydrocarbon group formation and better elongation in treated polymers. Everyday applications benefit from enhanced hydrophilic features, color strength, and antimicrobial properties in fabrics and packaging.
Conclusion
Understanding crosslinking and chain scission in electron beam processing helps industries achieve stronger, more durable polymers with less environmental impact.
| Key Benefit | Description |
|---|---|
| Enhanced Performance | Stronger bonds improve strength and durability. |
| Environmental Advantages | Reduced chemical use and energy consumption. |
| Faster Processing | Shorter production cycles for many applications. |
Precise control of processing conditions ensures optimal results. Ongoing research will unlock new possibilities for advanced material design.
FAQ
What Is the Main Difference Between Crosslinking and Chain Scission?
Crosslinking creates strong bonds between polymer chains. Chain scission breaks chains into smaller pieces.
Crosslinking increases strength. Chain scission reduces molecular weight.
How Does Electron Beam Dose Affect Polymer Properties?
Higher electron beam doses increase chain scission, making polymers brittle. Lower doses favor crosslinking, improving strength and durability.
| Dose Level | Main Effect |
|---|---|
| Low | Crosslinking |
| High | Chain Scission |
Can Crosslinking and Chain Scission Occur Together?
Both reactions can happen at the same time during electron beam processing. The balance depends on polymer type and processing conditions.
- Crosslinking and chain scission compete
- Engineers adjust settings for desired results
